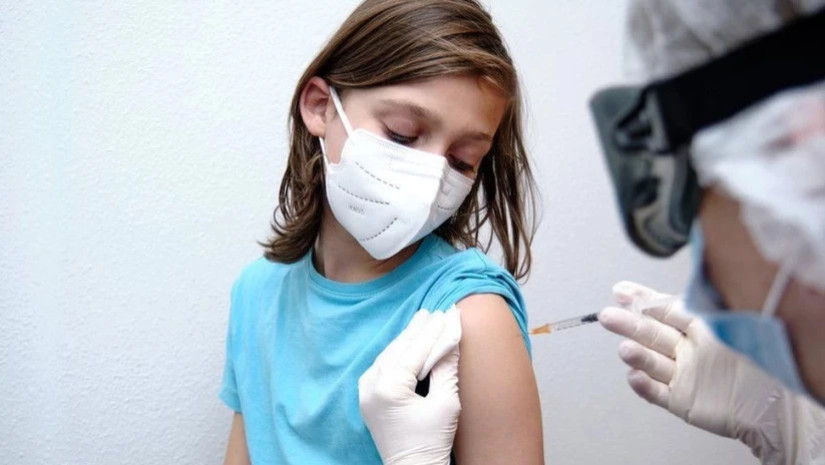
Parents won't be able to vaccinate their healthy children 6 months to 5 years old if the FDA pulls Pfizer's COVID-19 vaccine authorization.
The Food and Drug Administration may revoke authorization for Pfizer’s COVID-19 vaccine for healthy children under age 5, the pharmaceutical company confirmed, which would limit parents' vaccine options ahead of the winter respiratory virus season.
The possibility comes several months after President Donald Trump’s Department of Health and Human Services began placing limits on COVID-19 vaccines. For the last four years, updated COVID-19 vaccines have been made available in the fall for most Americans before the cold sets in.
The federal agency told Pfizer that it might not renew the emergency use authorization, or EUA, for the Pfizer-BioNTech COVID-19 vaccine Comirnaty for children ages 6 months through 4 years, according to a statement sent to USA TODAY.
“We are currently in discussions with the agency on potential paths forward and have requested that the EUA for this age group remain in place for the 2025-2026 season,” a company spokesperson said. “It is important to note that these deliberations are not related to the safety and efficacy of the vaccine, which continues to demonstrate a favorable profile.”
In July, Moderna received full FDA approval for its COVID-19 vaccine for children 6 months to 11 years old who are at increased risk of contracting COVID-19. The vaccine, Spikevax, is expected to be available for eligible populations in the 2025-26 respiratory virus season.
The Centers for Disease Control and Prevention still recommends older patients get vaccinated against COVID-19, as well as people whose immune systems have been weakened by illness or medical treatments such as chemotherapy.
But if Pfizer loses its EUA, parents won't have the option to vaccinate their healthy young children.